Which Best Defines Partial Pressure in a Mixture of Gas
Each constituent gas in a mixture of gases has a partial pressure which is the notional pressure of that constituent gas if it alone occupied the whole volume of the original mixture at the same temperature. Meaning of partial pressure.
Dalton S Law Of Partial Pressure Article Khan Academy
This contribution is the partial pressure.
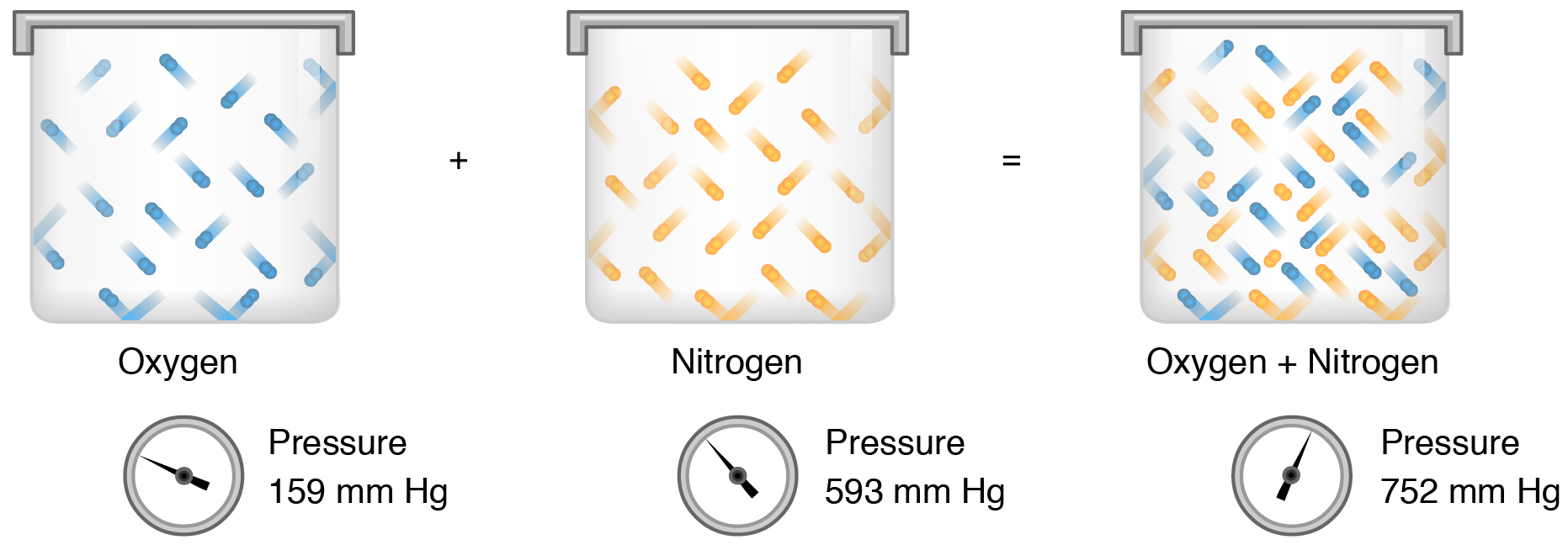
. Our partial pressure equation becomes P total P nitrogen P oxygen P carbon dioxide. O pressure that is exerted by all the gases of a mixture on the container. The partial pressure of the CO2 is 24 kPa and the partial pressure of the N2 is 48 kPa.
In a mixture of gases each gas contributes to the total pressure of the mixture. AnswerPressure that is exerted by one gas as if it occupied a container by itselfExplanationPartial pressure refers to the pressure exerted by an i. If the partial pressure of hydrogen is 1 atm find the mole fraction of oxygen in the mixture.
The pressure that each gas In a mixture would exert alone ----- law states that the total pressure of a gas mixture Is equal to the sum of the Individual partial pressures of gases. Which best defines partial pressure in a mixture of gases. Assuming ideal behavior what is the partial pressure.
Which best defines partial pressure. The overall pressure of an ideal gas mix is. Daltons law the statement that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual component gases.
Boyles Law and the Ideal Gas Law tell us the total pressure of a mixture depends solely on the number of moles of gas and not the kinds of molecules. By soetrust December 3 2021 Leave a reply 15. Which best defines partial pressure in a mixture of gases.
A mixture of hydrogen gas and oxygen gas exerts a total pressure of 15 atm on the walls of its container. The partial pressure of one gas in a mixture of gases contained in a given volume is the pressure that that one gas would exert if it occupied the entire volume all by itself. The best definition is pressure that is exerted by one gas as if it occupied a container by itself.
P He mm Hg P CH 4 mm Hg. This law states that. Partial pressure is a proxy measurement of mole fraction andor volume fraction in a gas mixture.
The partial pressure is the pressure that each gas would exert if it alone occupied the volume of. The partial pressure of an individual gas is equal to the total pressure multiplied by the mole fraction of that gas. The pressure that each gas in a mixture would exert alone.
We need to find the partial pressure for each gas and the total pressure the gas mixture exerts in the container. Keep this always in mind --- Partial pressure is most often not intended to. There are 10g of each gas and the temperature of each gas in the flask is 37 degrees C 986 degrees F.
Pressure that is exerted by all the gases of a mixture on the container pressure that is exerted by one gas as if it occupied a container by itself half of the pressure that is exerted by the gases of a mixture on the container sum of the. Were doing our. Half of the pressure that.
What is the mole fraction of each gas in the mixture. Applying Daltons law formula P total P hydrogen P oxygen. This is indeed the most important partial pressure gas law considered so far.
X N 2 X CO 2. The partial pressure of a single gas is proportional to the percentage of the gas in a mixture of gases. Up to 256 cash back What is the partial pressure of each gas in the mixture.
Which best defines partial pressure in a mixture of gases. Which best defines partial pressure. Daltons law states the total pressure of a mixture of ideal gases is the sum of the partial pressure of each individual gas.
V Volume of the whole gas mixture. 1 question Which best defines partial pressure in a mixture of gases. Now the mole fraction of oxygen X oxygen P oxygen P total 0515 033.
The amount of the gas dissolved in a certain liquid and the partial pressure of the gas accumulated above the surface of that liquid are proportional to each other Partial Pressure Equation. Partial pressure is the pressure that an individual gas exerts in a mixture of gases which in distillation can have an effect on boiling so pressure may have to be increased to achieve the boiling temperature. The total pressure of gas mixture nx is the amount of substance of a gas ntot is the total amount of substance in gas mixture The partial pressure of a gas is a measure of thermodynamic activity of the gass molecules.
Pressure that is exerted by one gas as if it occupied a container by itself. 2 A mixture of nitrogen and carbon dioxide gases contains nitrogen at a partial pressure of 363 mm Hg and carbon dioxide at a partial pressure of 564 mm Hg. Daltons Law allows us to calculate the total pressure in a system from each gas individual contribution.
_____ law states that the amount of gas dissolved in water is determined by its solubility in the fluid and the partial pressure of the gas in the surrounding air. The partial pressure is the pressure the gas if the gas were in the same volume and temperature by itself. A mixture of gases at a total pressure of 95 kPa contains N2 CO2 and O2.
The concentration 𝑛𝑉 of the mixture is found to be 0150 molL. O pressure that is exerted by all the gases of a mixture on the container o pressure that is exerted by one gas as if it occupied a container by itself o half of the pressure that is exerted by the gases of a mixture on the container sum of the individual pressures that are exerted by two. The reason that partial pressure equals final pressure when all other gasses are removed is because at that point volume and mole fraction equal 1 so Ptotal pO 2.

Dalton S Law Of Partial Pressure Article Khan Academy

How Do The Partial Pressures Of Gases In A Mixture Affect Each Other Quora

Comments
Post a Comment